

A battery is made up of a positive electrode (cathode), negative electrode (anode), and an electrolyte to allow the flow of electrical charge between the two electrodes.
In a conventional lithium-ion battery cell, the negative electrode is usually made from carbon. The positive electrode is a metal oxide, and the electrolyte is a mixture of lithium salt and organic solvent.
To make a battery viable as an electric storage device, eight basic requirements must be met. These are as follows.
Possible 9th and 10th requirements could be having low self-discharge to allow long shelf life with minimal performance degradation over time, and providing an instant start-up when needed.
Lithium-ion batteries are referenced by their active chemical makeup (e.g. lithium cobalt oxide has the chemical symbols LiCoO2 and can be abbreviated to LCO or Li-cobalt). A list of commonly used chemistries are described below.
Common Uses: Mobile phones, laptops and digital cameras.
Li-cobalt has high energy density, a relatively short life span, low thermal stability (safety risk – especially when damaged) and limited load capabilities (specific power). Newer systems include nickel, manganese and/or aluminum to improve longevity, loading capabilities and cost.
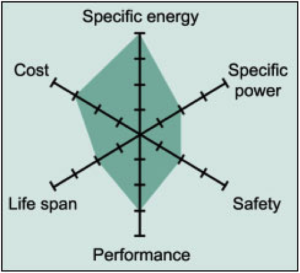
Common Uses: Power tools, medical instruments, hybrid/electric vehicles (e.g. Nissan Leaf, Chevy Volt and BMW i3)
High thermal stability and enhanced safety, but life is limited. Low internal cell resistance enables fast charging and high-current discharging. Pure Li-manganese batteries are no longer common today; with most now blended with lithium nickel manganese cobalt oxide (NMC) to improve the specific energy and prolong the life span.
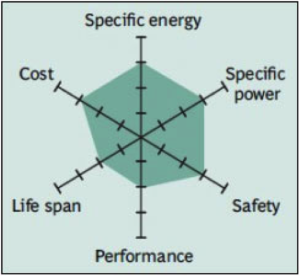
Common Uses: power tools, e-bikes, automotive, and energy storage systems (ESS).
One of the most successful Li-ion systems is a cathode combination of nickel-manganese-cobalt (NMC). The secret of NMC lies in combining nickel and manganese. While toxic on their own, mixing them serves to enhance each other strengths. NMC offers lower energy density, but a longer life and inherent safety with the lowest self-heating rate.
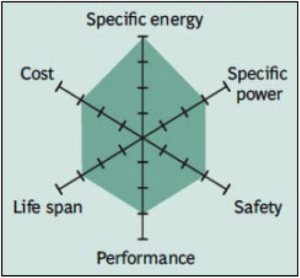
Common Uses: bicycles, electric cars, solar lighting, and energy storage systems (ESS).
The key benefits are long cycle life, good thermal stability, enhanced safety and tolerance if abused. Li-phosphate is more tolerant to full charge conditions and is less stressed than other lithium-ion systems if kept at high voltage for a prolonged time, but offers lower energy density.
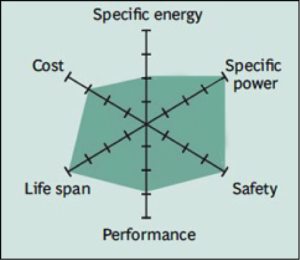
Common Uses: specialty designs aimed at particular niche roles.
Lithium nickel cobalt aluminum oxide shares similarities with NMC by offering high specific energy, reasonably good specific power and a long life span. Less flattering are safety and cost. NCA is a further development of lithium nickel oxide; adding aluminum gives the chemistry greater stability.
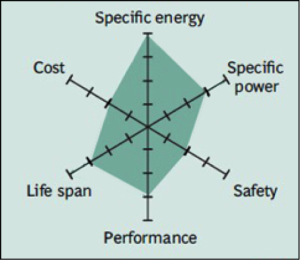
Common Uses: electric powertrains, UPS and solar-powered street lighting.
Li-titanate is safe and has excellent low-temperature discharge characteristics; however the battery is very expensive and has lower specific energy.
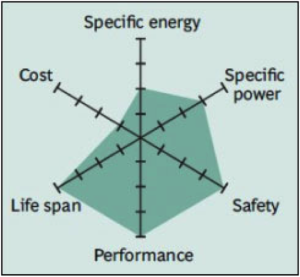
For more info: Battery University




Living off the grid has always been a popular choice for individuals who want to live self-sufficiently and sustainably. However, the concept of off-grid living

Australia, with its vast landscapes and abundant sunshine, has always been an agricultural powerhouse. However, the challenges faced by farmers and agribusinesses today, from rising

As a way of showing our appreciation for any new customers that you send our way, we’re launching a referral rewards program

Our experience with the Bulk Buy Project and building our home with power from the off-grid solar system we had installed on the existing garage.
Off-Grid Energy Australia · Securing your power and your future
Electrical Contractor Licenses: VIC REC-31913, TAS 15608294, WA EC15901, SA PGE278927, NSW 279181C